Home > Research themes > Development of an efficient method for producing fully human monoclonal antibodies for clinical application
Main content starts here.
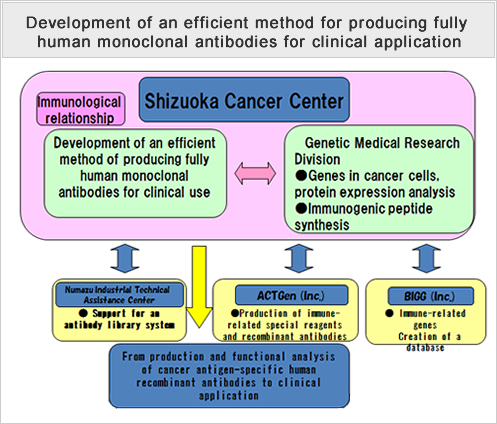
Development of an efficient method for producing fully human monoclonal antibodies for clinical application
Yasuto Akiyama, Chief of Immunotherapy Division, Shizuoka Cancer Center Research Institute
(FY2010-2012)
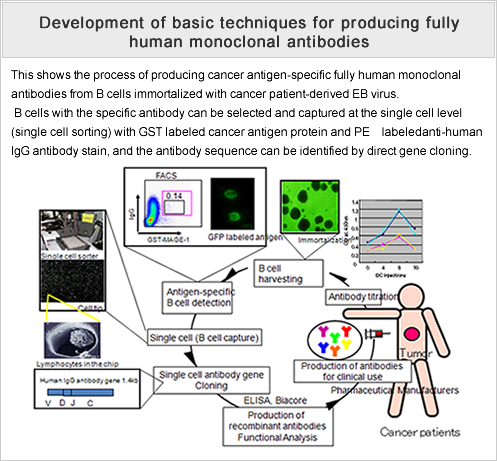
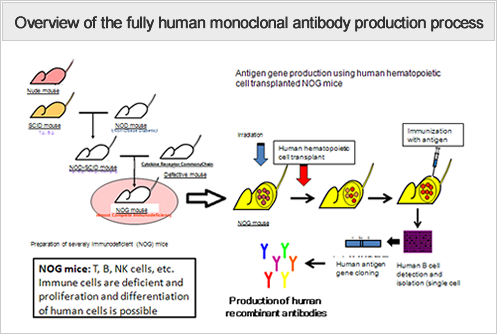
We will conduct research and development aimed at producing cancer antigen-specific, functional, fully human antibodies by immunizing highly immunodeficient mice transplanted with a human immune system with antigen protein.
In order to further streamline the process of producing recombinant antibodies, we will combine our research with development of novel antibody purification techniques that do not use serum-free culture systems of suspended CHO cells or protein A/G carrier antibodies.
FY2010 results
- We have almost established a recombinant protein production technique and human antibody production system transplanted with umbilical cord blood cells in NOG mice according to our initial plan.
- We discovered a novel affinity ligand to human antibodies and made a patent application.
- 1 We made a PCT international application for a method of analyzing and identifying antibody genes at the cellular level and put together seven related papers.


