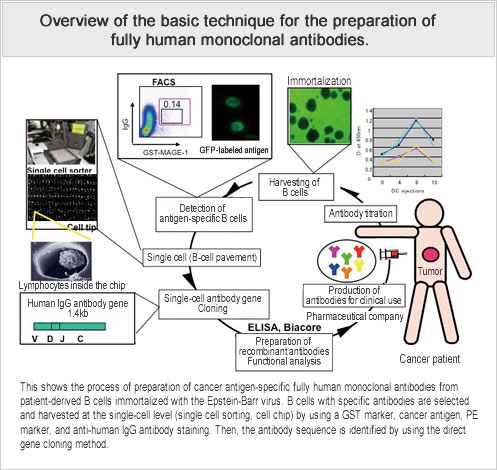
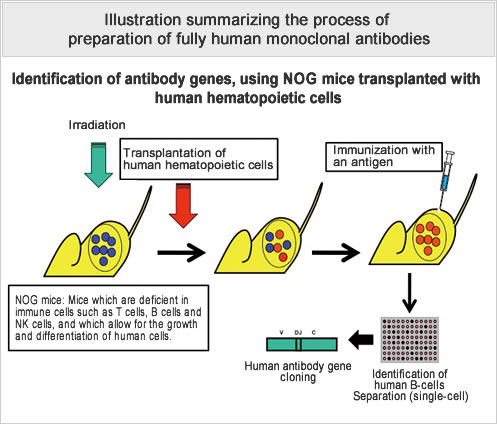
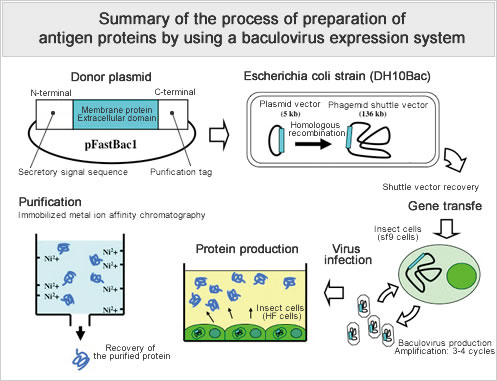
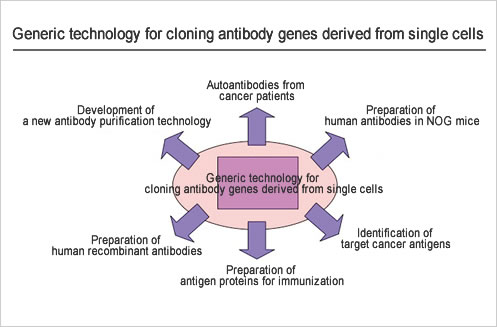
Home > Research themes > Development of an efficient method for producing fully human monoclonal antibodies for clinical application
Main content starts here.
Development of an efficient method for producing fully human monoclonal antibodies for clinical application
Yasuto Akiyama, Chief of Immunotherapy Division, Shizuoka Cancer Center Research Institute
(FY2010-2012)
Aims and Expected Results of Research
In the field of cancer treatment, where there is a need for a wide variety of therapeutic antibodies, an efficient system for the production of cancer antigen-specific and highly active human antibodies, needs to be established. The aim of this study is to establish a technique for the producton of cancer antigen-specific and functional fully human antibodies, on the basis of antibody production techniques used thus far, and by immunizing antigen proteins into severely immunocompromised mice transplanted with human antibody-producing cells. In addition, based on the antibody genes obtained from this study, we also plan to expand our research to the development of new antibody purification technology aimed at promoting the efficiency of the recombinant antibody production process.
Progress made during previous years
We constructed a human antibody production system using umbilical cord blood cells in severely immunocompromised mice, and found antibody titers confirming an induction of antibodies against specific cancer antigens.
FY2012 results
- By transplanting human antibody-producing cells into immunodeficient mice, and by immunizing the mice with cancer antigen proteins, we have established a technique for the production of cancer antigen-specific human monoclonal antibodies.
- By creating full-length human (chimeric) antibodies through the use of a baculovirus expression system, we have successfully produced functional antibodies.
In the future, we will proceed to the expansion of their applications, on the basis of the outcomes of the development of "basic techniques established thus far for the cloning of antibody genes from single cells".